Research in our laboratory is focused on insect chemosensory systems, particularly mechanisms supporting robust responses to odors. We are currently interested in how the molecular and cellular environment surrounding olfactory neurons influences their activity, and odor-driven behaviors. Given that olfaction plays an essential role in insect behavior, including the attraction of insect vectors of disease to human hosts, we expect that our research will both reveal fundamental principles of sensory neuroscience and contribute to human health.
Why study Drosophila?
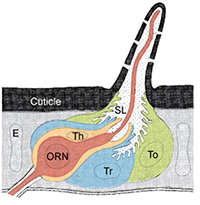
Many features of insect olfactory systems are conserved. The molecular basis of insect olfaction is best characterized for Drosophila due to its status as a genetic model organism. Odors are detected by olfactory receptor neurons (ORNs) located on insect antennae inside sensory hairs known as sensilla. In Drosophila, olfactory sensilla have been classified into functional classes based on their odor response profiles. A receptor to neuron map has been generated, revealing stereotyped combinations of odorant receptors in each functional class. Our lab takes advantage of this well-characterized organization and the enormous genetic toolbox in Drosophila to make molecular manipulations of targeted sensillar and cellular populations.
Auxiliary cells may have a conserved role in olfactory signaling
ORNs are encompassed by non-neuronal auxiliary cells, whose contribution to olfaction is largely unexplored in both insects and vertebrates. Auxiliary cells are thought to play a major role in odor clearance from the extracellular peri-neuronal space (sensillar lymph) and may contribute to signal termination. Odors diffuse slowly out of aqueous olfactory tissues compared to the rate of environmental fluctuations. Rapid odor uptake or degradation by auxiliary cells may allow ORNs to faithfully transmit temporal information about odor stimuli.
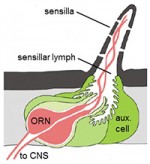
What is the relationship between auxiliary-cell mediated odor uptake and ORN odor responsiveness?
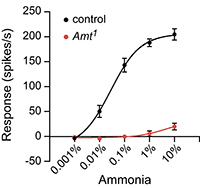
In a screen for genes with roles in olfactory signaling, we identified an auxiliary cell transporter, Amt. Amt is highly conserved evolutionarily and likely mediates ammonia uptake from the sensillar lymph. We discovered that ORN responses to ammonia are nearly abolished in Amt1 flies, providing one of the first clear examples of an auxiliary cell protein making a profound and specific contribution to ORN activity. Ongoing work in the lab uses ammonia detection as a model system to determine the mechanism by which odor clearance has such a strong impact on ORN activity.
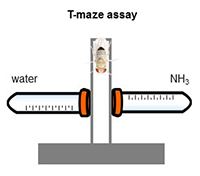
What molecules support behavioral attraction to ammonia?
Ammonia is an attractive cue for many insect vectors of human disease, including malaria-bearing mosquitoes. Thus the molecules that support olfactory sensitivity to ammonia could serve as targets for novel insect repellents. Our lab is testing the behavioral significance of genetic Amt disruption and exploring whether other conserved molecules support ammonia sensitivity through the generation of new mutants using CRISPR engineering.
What molecules support odorant clearance more generally?
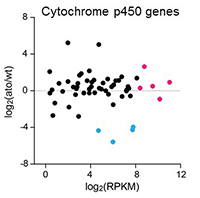
Given the vast variety and number of odors, additional mechanisms for odor removal must exist beyond odor specific transporters. Notably high levels of xenobiotic metabolizing enzymes have been reported in the olfactory tissues of numerous insect and vertebrate species. Expression of these molecules suggests that auxiliary cells may prevent toxic volatile substances from damaging olfactory tissues, but their contribution to ORN activity in vivo is mostly unknown. Using next generation sequencing (RNASeq), we identified several candidate genes whose physiological and behavioral function we are now examining. We are also using bioinformatics to identify which genes may have a conserved role in olfaction across insect species.
What distinguishes the three types of auxiliary cells?
Adult olfactory sensilla contain three types of auxiliary cells that are treated as if functionally equivalent, a consequence of the poor characterization of their molecular makeup. Our lab will be using cell-type specific gene profiling to compare gene expression between tormogen, trichogen, and thecogen auxiliary cells. This work will provide insight into the unique functions of these cells in the olfactory system and open up future avenues of research probing the influence of auxiliary cells on olfactory signaling. This research may also have implications for the physiology of other insect sensory systems because these three cell classes are also found in many other types of sensory sensilla.